#KC1-5 What Happens When We Age? Part 1/3
Antagonistic Hallmarks - Cellular Senescence
After talking about the Primary Hallmarks in the last four posts, we're now moving on to the Antagonistic Hallmarks. "Antagonistic" essentially means "acting against." These hallmarks are the responses to those primary causes we discussed earlier.
In our post on Telomere Attrition, we introduced telomeres as protective caps at the ends of our DNA. If you recall, we mentioned that when telomeres are reduced to a critical length, a cell's genome becomes too unstable for further division. As a protective measure, division will be permanently turned off in these cells to halt the spread of damaged DNA. The process of cells entering this permanent nondividing state is called Cellular Senescence. Here, telomere attrition is just one example; there are other types of damage that we haven’t discussed yet, which can also lead to this state.
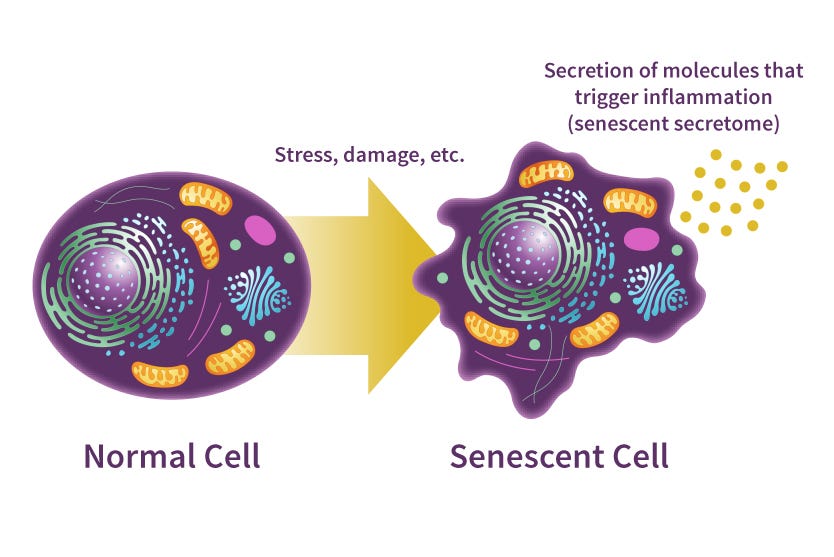
Through evolution, our bodies have developed a smart innate maintenance system to care for these senescent cells. Once cells enter this state, they begin releasing specific inflammatory molecules, triggering an immune response. This activation prompts our immune system to jump into action, clearing out these senescent cells — much like how our bodies handle germs and viruses when we're under the weather. This entire process, first, effectively prevents these senescent cells from passing on their damaged DNA through division or becoming cancerous (that uncontrollable division and growth commonly seen in malignant tumor cells) by permanently turning off division. And then, it efficiently removes and recycles these damaged cells by engaging our immune system. Truly an impressive strategy!
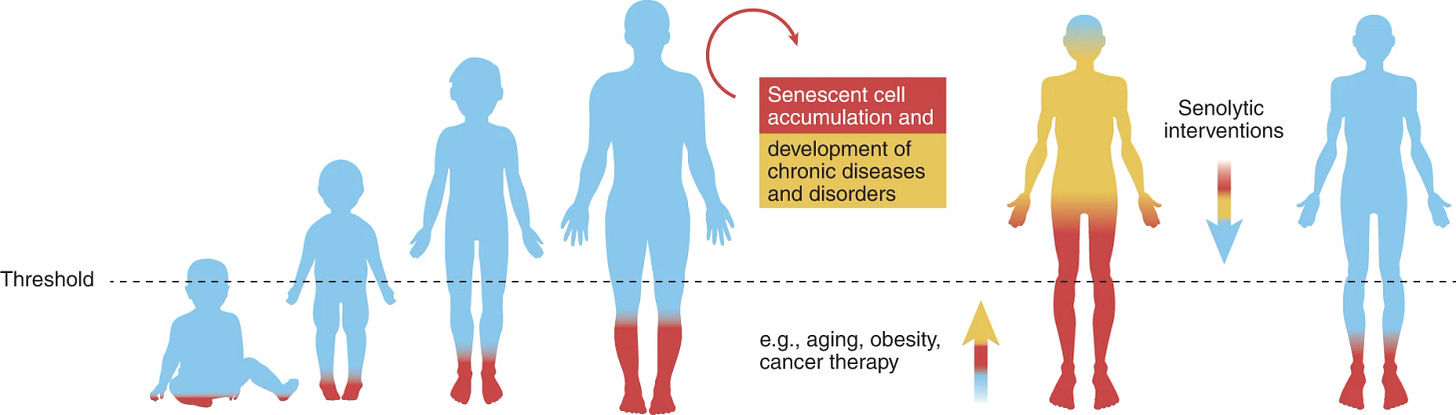
As we age, however, this process starts losing its efficiency due to those Primary Hallmarks (the causes of aging) we discussed in earlier posts. The waiting line just keeps growing longer and longer. More and more senescent cells end up spending extended periods in this senescent state. If these senescent cells could just chill and enjoy their retirement in peace, we wouldn't be grappling with any major issues. Unfortunately, without efficient clearance, these senescent cells keep pumping out inflammatory molecules, leading to a surge of chronic inflammation within our bodies. These inflammatory molecules also spread to our healthy neighboring cells, causing them to malfunction and disrupting the regular operation of our tissues and organs. One of the common consequences of this issue is the gradual stiffening of the walls of our heart's blood vessels, causing them to lose the flexibility they need for their proper function. As these changes progress, the hardening of arteries can lead to age-related heart disease and increase our risks of future heart attacks down the road.
Sadly, cellular senescence doesn’t take place in just one organ; it happens at the cellular level across various tissues and organs. According to Dr. David Sinclair in his book "Lifespan," those inflammatory molecules can even stimulate surrounding cells to become cancerous, escalating the issue further.
Over the past few years, you've probably heard a lot in the media about the dark side of chronic inflammation. Due to its significant role in aging research, scientists have given it a specific name — "Inflamm-aging." Chronic inflammation has been recognized as a driving force behind many well-known aging-related diseases, such as heart disease, diabetes, Alzheimer's disease, cancers, and more. We'll delve deeper into these conditions and explore ways to prevent them at the cellular level in future posts.
What We Can (potentially) Do
A class of pharmaceuticals called senolytics can target and kill senescent cells as they should if our clearance mechanism works properly. Dr. James Kirkland and his team from the Mayo Clinic discovered that by giving a short course of two senolytic molecules, quercetin and dasatinib, to enhance the elimination of senescent cells in lab mice, the lifespan of the mice was extended by a remarkable 36%. Moreover, they managed to reverse some severe aging-related diseases in mice, including different models of Alzheimer's disease.
Data from the first published in-human trial, a pilot study involving patients with Idiopathic Pulmonary Fibrosis (a fatal disease associated with cellular senescence), as well as preliminary results from a clinical trial on patients with diabetic kidney disease, conducted by Dr. Kirkland's team, have also indicated promising outcomes so far.
More human clinical trials on senolytics are currently underway. It will be a few more years before we gain a better understanding of these drugs. Large, randomized controlled trials are currently being carried out or planned to evaluate and ensure the safe and effective dosing of senolytics. The findings from these trials will not only determine the long-term safety and efficacy of senolytics but could also have a transformative impact on the care and treatment of various aging-related diseases.
Another frequently mentioned drug is Rapamycin, which belongs to a class of drugs called senomorphics. These molecules don't kill senescent cells but do stop them from releasing inflammatory molecules. Rapamycin has been shown to prevent cognitive decline and accumulating Alzheimer's disease pathology across several studies using different mouse models. But again, while scientists are reasonably confident about its efficacy in humans, as of right now, there is still limited knowledge regarding the appropriate dosage for aging reversal purposes and its long-term consequences.
While we are waiting to determine the optimal dosage and the most effective drug for the job, let’s not forget that cellular senescence is one of the responses to the Primary Hallmarks of Aging. Following the recommended practices mentioned in previous Primary Hallmarks posts can alleviate stress on our senescent cell clearance mechanism at its core. These combined efforts will help keep our bodies in good shape and prepare us for when pharmaceutical treatments become available.
The Takeaway
As cells accumulate damage over time, they will eventually reach a state where they become too unstable to undergo further division. This phenomenon is known as Cellular Senescence. Apart from losing their own functions, if these senescent cells aren't efficiently cleared, the inflammatory molecules they release will contribute to chronic inflammation and further damage the surrounding tissue, resulting in a more extensive issue within our bodies.
Pharmaceutical research is currently in progress to find drugs that can address this senescent cell problem. Although the pilot study and preliminary data have shown promise, it's important to be cautious since the long-term safety and optimal dosage are still being explored.
Next post, we will take a short break from the Hallmarks of Aging series and talk about another interesting concept. This concept will offer us a fresh and enlightening perspective to better understand these hallmarks and their mechanisms along the way. Stay tuned!


Being, now, a 64 year old boomer this topic greatly interests me. So a few years back I read a book about aging 8forget the title) btu the author recomended taking NMN & Resveratrol as a way of slowing the aging process. So I was wondering if you have any thoughts on that.
Thanks